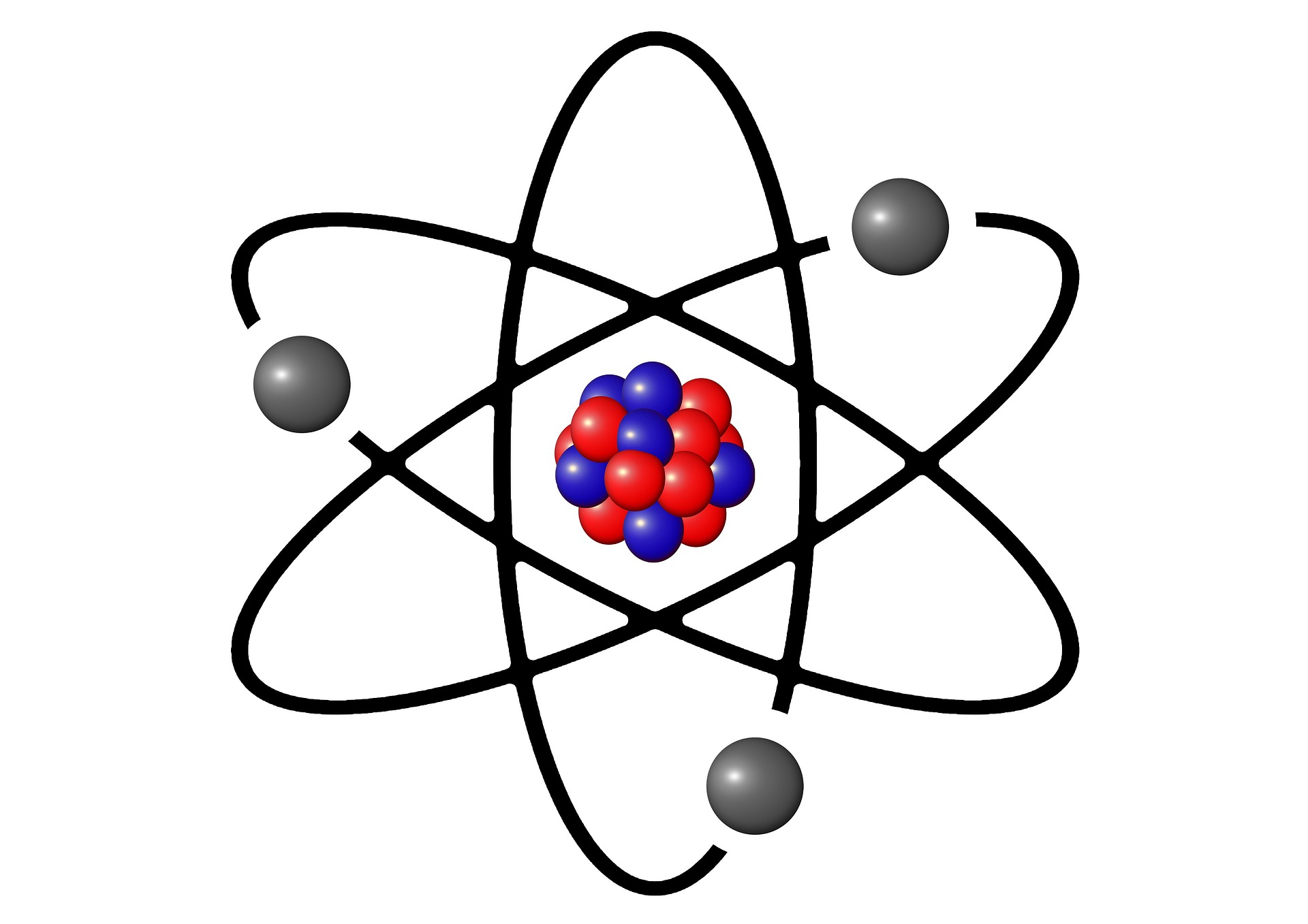
An atom is the basic building block of matter, the smallest unit of an element that retains the chemical properties of that element. Atoms are incredibly small, with a size on the order of 0.1 to 0.5 nanometers (1 nanometer = 10⁻⁹ meters). They consist of subatomic particles and are the foundation of all physical substances in the universe.
Key Properties
- Atomic Number: The number of protons, which defines the element (e.g., oxygen has 8 protons).
- Mass Number: The total number of protons and neutrons.
- Isotopes: Variants of an element with the same number of protons but different numbers of neutrons (e.g., Carbon-12 and Carbon-14).
- Charge: Atoms are typically neutral because the number of protons equals the number of electrons. Ions form when this balance is disrupted.
Discovery and History of the Atom
The concept of the atom has evolved over centuries through scientific discoveries. The idea of an atom was first proposed by the Greek philosopher Democritus (460–370 BCE), who believed that all matter was composed of tiny, indivisible particles called “atomos.” However, this idea remained speculative until modern science provided experimental evidence
In 1803, John Dalton proposed the first scientific atomic theory. He stated that atoms are indivisible, combine in fixed ratios to form compounds, and retain their identity in chemical reactions. Dalton’s theory laid the foundation for modern chemistry.
In 1897, J.J. Thomson discovered the electron using a cathode ray tube, proving that atoms are divisible and contain smaller charged particles. He proposed the “plum pudding model,” where negatively charged electrons were embedded in a positively charged sphere.
In 1911, Ernest Rutherford conducted the gold foil experiment, discovering that atoms have a small, dense, positively charged nucleus surrounded by empty space and orbiting electrons. This overturned Thomson’s model.
In 1913, Niels Bohr refined Rutherford’s model, introducing the concept of electron shells or energy levels, stating that electrons move in fixed orbits around the nucleus.
In 1932, James Chadwick discovered the neutron, completing the modern understanding of atomic structure.
Parts of an Atom
An atom is composed of three primary subatomic particles:
- Protons: Positively charged particles found in the nucleus (the central part of the atom). The number of protons (atomic number) determines the element (e.g., 1 for hydrogen, 6 for carbon).
- Neutrons: Neutral particles (no charge) also located in the nucleus. The combined number of protons and neutrons is the atom’s mass number.
- Electrons: Negatively charged particles that orbit the nucleus in electron shells or energy levels. Electrons are involved in chemical reactions and bonding.
The nucleus, containing protons and neutrons, is extremely dense and accounts for nearly all of an atom’s mass, while the electron cloud surrounding it determines its size and chemical behaviour.
The number of protons in an atom determines its atomic number, while the sum of protons and neutrons determines its atomic mass. Electrons balance the positive charge of protons, making the atom electrically neutral.
Atomic Structure
The structure of an atom consists of:
- Nucleus – The core of the atom containing protons and neutrons. It holds most of the atom’s mass.
- Electron Shells – Electrons are arranged in energy levels around the nucleus. The first shell holds up to 2 electrons, the second up to 8, and so on, following the 2n² rule.
- Subatomic Forces – The nucleus is held together by the strong nuclear force, while electrons are attracted to the nucleus due to the electromagnetic force.
Important Atomic Theories
- Dalton’s Atomic Theory (1803)
- Atoms are indivisible and indestructible.
- Atoms of the same element are identical.
- Atoms combine in simple ratios to form compounds.
- Thomson’s Model (1897)
- Atoms are made of a positive sphere with embedded negative electrons (plum pudding model).
- Rutherford’s Model (1911)
- Atoms have a dense, positively charged nucleus.
- Electrons move around the nucleus in empty space.
- Bohr’s Model (1913)
- Electrons move in fixed orbits with quantized energy levels.
- Quantum Mechanical Model (1926)
- Proposed by Schrödinger, this model describes electrons as probability clouds rather than fixed orbits.
Conclusion
- The discovery of the atom and its structure revolutionized science, leading to advancements in nuclear energy, chemistry, medicine, and technology. The understanding of atomic behavior continues to shape innovations in quantum mechanics, nanotechnology, and space exploration.